Carbonate and phosphite encaged in frameworks constructed from square lanthanum aminopolycarboxylates and sodium chloride†
Abstract
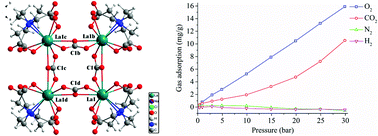
Novel additives of lanthanum aminopolycarboxylates with inorganic anions, Na12n[La(edta)L]4n·8nNaCl·4nH2O (1: L = HPO32−; 2: L = CO32−) and K12n[La(cdta)(CO3)]4n·35nH2O (3) (H4edta = ethylenediaminetetraacetic acid; H4cdta = cyclohexanediaminetetraacetic acid), were obtained in alkaline solution. Structural analyses reveal that 1 and 2 are isomorphous and contain interesting square structures. HPO32− (CO32−) was encaged in the constructed tetranuclear frameworks. Tetranuclear lanthanum ethylenediaminetetraacetate was further encaged in superstructures of sodium chloride. 3 has a similar square structure, in which edta is replaced by cdta. All complexes are fully characterized via elemental, FT-IR, NMR, thermogravimetric and structural analyses. Solution 13C NMR spectra show that 1 and 2 dissociate into mononuclear units in water. Interestingly, 2 possesses 3.7 Å diameter holes inside its crystals, which can adsorb a small amount of O2 or CO2 selectively. The amounts of O2 and CO2 adsorbed increase gradually from 0.32 and 0.38 mg g−1 at 0.4 bar to 15.90 and 10.54 mg g−1 at 29.9 bar, respectively.


 Please wait while we load your content...
Please wait while we load your content...