Synthesis and catalytic activity of tridentate N-(2-pyridylethyl)-substituted bulky amidinates of calcium and strontium†
Abstract
Metalation of the formamidine Dipp–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(H)–N(H)–C2H4–Py (1a) and benzamidine Dipp–N
C(H)–N(H)–C2H4–Py (1a) and benzamidine Dipp–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(Ph)–N(H)–C2H4–Py (1b) with [(thf)2M{N(SiMe3)2}2] (M = Ca, Sr) yields the corresponding homoleptic complexes [M{Dipp–N
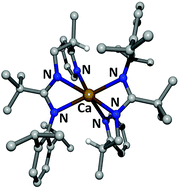
C(Ph)–N(H)–C2H4–Py (1b) with [(thf)2M{N(SiMe3)2}2] (M = Ca, Sr) yields the corresponding homoleptic complexes [M{Dipp–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(R)–N–C2H4–Py}2] (M/R = Ca/H (2a), Ca/Ph (2b), and Sr/Ph (3b)) regardless of the applied stoichiometry. Only during calciation of Dipp–N
C(R)–N–C2H4–Py}2] (M/R = Ca/H (2a), Ca/Ph (2b), and Sr/Ph (3b)) regardless of the applied stoichiometry. Only during calciation of Dipp–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(H)–N(H)–C2H4–Py (1a), the heteroleptic intermediate [{(Me3Si)2N}Ca{Dipp–N
C(H)–N(H)–C2H4–Py (1a), the heteroleptic intermediate [{(Me3Si)2N}Ca{Dipp–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(R)–N–C2H4–Py}]2 (2a′) has been observed. The formamidinate complex of strontium crystallizes as a tmeda adduct of the type [(tmeda)Sr{Dipp–N
C(R)–N–C2H4–Py}]2 (2a′) has been observed. The formamidinate complex of strontium crystallizes as a tmeda adduct of the type [(tmeda)Sr{Dipp–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(H)–N–C2H4–Py}2] (3a). Metalation of the pivalamidine Dipp–N
C(H)–N–C2H4–Py}2] (3a). Metalation of the pivalamidine Dipp–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(tBu)–N(H)–C2H4–Py (1c) leads to the formation of heteroleptic mononuclear [{(Me3Si)2N}M{Dipp–N
C(tBu)–N(H)–C2H4–Py (1c) leads to the formation of heteroleptic mononuclear [{(Me3Si)2N}M{Dipp–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(tBu)–N(H)–C2H4–Py}] (M = Ca (2c) and Sr (3c)) with a side-on bonding of the Dipp group to the alkaline earth metals. Calciation of chiral Dipp–N
C(tBu)–N(H)–C2H4–Py}] (M = Ca (2c) and Sr (3c)) with a side-on bonding of the Dipp group to the alkaline earth metals. Calciation of chiral Dipp–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(tBu)–N(H)–CH2CH(Me)–Py (R)-1d and (S)-1d with [(thf)2Ca{N(SiMe3)2}2] yields the homoleptic complexes [Ca{Dipp–N
C(tBu)–N(H)–CH2CH(Me)–Py (R)-1d and (S)-1d with [(thf)2Ca{N(SiMe3)2}2] yields the homoleptic complexes [Ca{Dipp–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(tBu)–N–CH2CH(Me)–Py}2] with the enantiomeric forms (R,R)-2d and (S,S)-2d regardless of the applied stoichiometry. The complexes 2c and 2d catalyze the intramolecular hydroamination of the aminoalkene 2,2-diphenylpent-4-enylamine yielding 2-methyl-4,4-diphenylpyrrolidine but the stereochemistry cannot be influenced by the chiral compounds (R,R)-2d and (S,S)-2d.
C(tBu)–N–CH2CH(Me)–Py}2] with the enantiomeric forms (R,R)-2d and (S,S)-2d regardless of the applied stoichiometry. The complexes 2c and 2d catalyze the intramolecular hydroamination of the aminoalkene 2,2-diphenylpent-4-enylamine yielding 2-methyl-4,4-diphenylpyrrolidine but the stereochemistry cannot be influenced by the chiral compounds (R,R)-2d and (S,S)-2d.


 Please wait while we load your content...
Please wait while we load your content...