POCN Ni(ii) pincer complexes: synthesis, characterization and evaluation of catalytic hydrosilylation and hydroboration activities†
Abstract
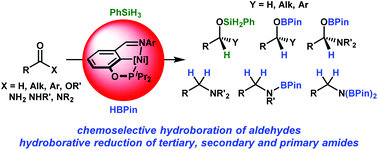
A series of iminophosphinite POCN pincer Ni(II) complexes, (POCN)NiMe and (POCN)NiLn(BX4) (L = CH3CN, n = 0, 1; X = F, Ph, C6F5), have been developed and subjected to catalytic hydrosilylation of alkenes, aldehydes and ketones and hydroboration of carbonyl compounds. The stoichiometric reactivity of (POCN)NiMe and (POCN)Ni(BF4) with PhSiH3 and HBPin suggests that catalytic reactions proceed via the hydride intermediate (POCN)NiH. With regard to reactions with HBPin, efficient and mild hydroboration of a variety of carbonyl compounds, including highly chemoselective hydroboration of benzaldehyde in the presence of other common potent reductive functional groups, such as alkenes, alkynes, esters, amides, nitriles, nitro compounds and even ketones, and the first example of base metal catalyzed hydroboration of amides, including mild direct hydroborative reduction of primary and secondary amides to borylated amines were demonstrated for (POCN)NiMe.


 Please wait while we load your content...
Please wait while we load your content...