An efficient copper-catalyzed synthesis of symmetrical bis(N-arylbenzamide) selenides and their conversion to hypervalent spirodiazaselenuranes and hydroxy congeners†
Abstract
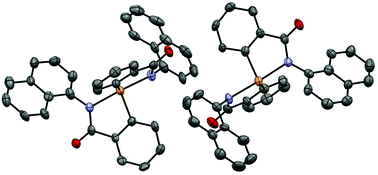
A copper catalyzed efficient synthetic method has been developed to access bis(N-arylbenzamide) selenides from 2-halo-N-arylbenzamide substrates and disodium selenide in HMPA at 110 °C. The developed protocol tolerates substituents in both N-aryl and benzamide rings of the 2-halobenzamide substrates and provides an array of bis(N-arylbenzamide) selenides in practical yields. The resulting selenides were transformed into hypervalent spirodiazaselenuranes by oxidation using aqueous hydrogen peroxide. (N-(1-Naphthyl)) spirodiazaselenurane is also structurally characterized by a single crystal X-ray study. Hydroxy-substituted spiroselenuranes have been prepared by careful demethylation of methoxy-substituted selenides followed by oxidation by hydrogen peroxide. Antioxidant properties for the decomposition of hydrogen peroxide and for the deactivation of radicals of hydroxy-substituted spiroselenuranes have been studied by the thiol assay and 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay. Both hydroxy-substituted spiroselenuranes exhibit dual mimic functions of glutathione peroxidase (GPx) selenoenzyme and α-tocopherol for decomposition of hydrogen peroxide and deactivation of radicals, respectively.
- This article is part of the themed collection: New Talent: Asia-Pacific


 Please wait while we load your content...
Please wait while we load your content...