Generation and reactivity of neutral 1,3-benzazaphosphole and anionic 1,3-benzazaphospholide ytterbium(iii) complexes†
Abstract
Treatment of Cp3Ln (Ln = Yb, Y) with 5-R3-6-R1-2-R2-1H-1,3-benzazaphosphole (HBp) (HBp1 (1a): R1 = H, R2 = 2,4,6-Me3C6H2, R3 = Me; HBp2(1b): R1 = Me, R2 = C6H5, R3 = H; HBp3(1c): R1 = R3 = H, R2 = C6H5) at room temperature gives the crystalline 1 : 1 Lewis acid–base adducts [(η1(p)-HBp)LnCp3] (2a–d) [Ln = Yb: Bp = Bp1 (2a), Bp2 (2b), Bp3 (2c); Ln = Y: Bp2 (2d)] with Ln–P donor bonds in good yields. Heating 2a–c in toluene leads to the liberation of one molecule of CpH to afford the corresponding N-bonded complexes [Cp2YbBp] (Bp = Bp1 (3a), Bp2 (3b), Bp3 (3c)). Interestingly, the P atom of complexes 3a–c can also be further coordinated to another Lewis acid such as Cp3Yb and B(C6F5)3 to give the adducts [Cp2Yb(μ–η1(N):η2(C,C):η1(P)-Bp)YbCp3] (Bp = Bp1 (4a), Bp2 (4b), Bp3 (4c)) and [Cp2Yb(μ–η1(N):η2(C,C):η1(P)-Bp)B(C6F5)3] (Bp = Bp1 (5a), Bp2 (5b), Bp3 (5c)), respectively. The molecular structures of complexes 2a, 4b–4c and 5c are confirmed by X-ray diffraction analysis.
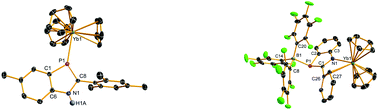


 Please wait while we load your content...
Please wait while we load your content...