Using para hydrogen induced polarization to study steps in the hydroformylation reaction†
Abstract
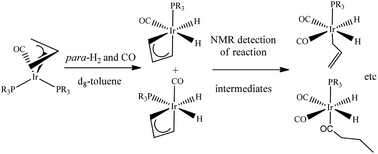
A range of iridium complexes, Ir(η3-C3H5)(CO)(PR2R′)2 (1a–1e) [where 1a, PR2R′ = PPh3, 1b P(p-tol)3, 1c PMePh2, 1d PMe2Ph and 1e PMe3] were synthesized and their reactivity as stoichiometric hydroformylation precursors studied. Para-hydrogen assisted NMR spectroscopy detected the following intermediates: Ir(H)2(η3-C3H5)(CO)(PR2R′) (2a–e), Ir(H)2(η1-C3H5)(CO)(PR2R′)2 (4d–e), Ir(H)2(η1-C3H5)(CO)2(PR2R′) (10a–e), Ir(H)2(CO–C3H5)(CO)2(PR2R′) (11a–c), Ir(H)2(CO–C3H7)(CO)2(PR2R′) (12a–c) and Ir(H)2(CO–C3H5)(CO)(PR2R′)2 (13d–e). Some of these species exist as two geometric isomers according to their multinuclear NMR characteristics. The NMR studies suggest a role for the following 16 electron species in these reactions: Ir(η3-C3H5)(CO)(PR2R′), Ir(η1-C3H5)(CO)(PR2R′)2, Ir(η1-C3H5)(CO)2(PR2R′), Ir(CO–C3H5)(CO)2(PR2R′), Ir(CO–C3H7)(CO)2(PR2R′) and Ir(CO–C3H5)(CO)(PR2R′)2. Their role is linked to several 18 electron species in order to confirm the route by which hydroformylation and hydrogenation proceeds.


 Please wait while we load your content...
Please wait while we load your content...