Catalytic coupling of CO2 with epoxide by metal macrocycles functionalized with imidazolium bromide: insights into the mechanism and activity regulation from density functional calculations†
Abstract
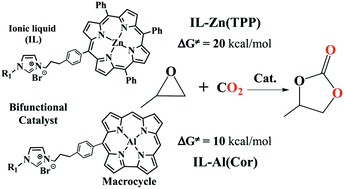
The cycloaddition of CO2 and epoxide catalysed by metalloporphyrins (IL-M(TPP)) and metallocorroles (IL-M(Cor)) containing imidazolium bromide has been studied extensively using density functional theory calculations. Possible mechanisms and catalytic effects of the hydrogen substitution on the imidazolium ring and the metal replacement in the macrocycles have been investigated. The results showed that the synergistic effect between the electrophilic metal centre and the flexible nucleophilic Br− of the bifunctional catalysts was responsible for the high catalytic activity. The coupling reaction of CO2 and ethylene oxide catalysed by IL-Al(Cor) experiences a free energy barrier of 9.7 kcal mol−1 for the rate-determining ring-opening step, which is much lower than ∼20 kcal mol−1 for that catalysed by IL-Zn(TPP). The metallocorrole-based bifunctional catalyst seems quite promising for the catalytic conversion of CO2 into five-membered heterocyclic compounds.


 Please wait while we load your content...
Please wait while we load your content...