In situ growth of metallic Ag0 intercalated CoAl layered double hydroxides as efficient electrocatalysts for the oxygen reduction reaction in alkaline solutions†
Abstract
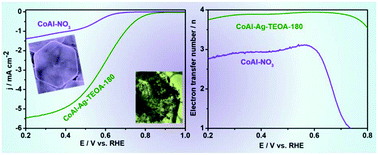
Metallic Ag0 intercalated CoAl-layered double hydroxides (CoAl LDHs) have been successfully synthesized in situ through a simple redox process with ethylene glycol (EG) and triethanolamine (TEOA). The Ag(CN)2− anion-exchanged precursor was reduced by EG to form metallic Ag0. Furthermore, the effect of TEOA on confining the particle size of Ag0 was demonstrated. The oxygen reduction reaction (ORR) property of metallic Ag0 intercalated CoAl LDHs was examined in alkaline aqueous solution. A typical sample synthesized by the addition of TEOA for 180 min exhibited excellent ORR catalytic activity with a high current density of 5.5 mA cm−2 at 0.2 V (vs. a reversible hydrogen electrode (RHE)) and good stability. Koutecky–Levich (K–L) calculations and rotating ring-disk electrode (RRDE) measurements further revealed that the ORR of the as-prepared catalyst proceeded mainly via an almost ideal four-electron transfer process. The enhanced electrocatalytic activity was ascribed to the intercalated Ag0, confined nanoparticle size and the expanded interlayer space, which effectively facilitate the reactant transfer and electron migration.


 Please wait while we load your content...
Please wait while we load your content...