Designing air-stable cyclometalated Fe(ii) complexes: stabilization via electrostatic effects†
Abstract
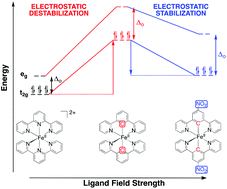
Designing efficient Fe(II) chromophores requires optimization of numerous, at times conflicting, properties. It has been suggested that replacement of polypyridine ligands with cyclometalated analogs will be effective at destabilizing the quintet state and therefore extending the lifetime of photoactive metal-to-ligand charge transfer states. However, cyclometalated Fe(II) complexes are not oxidatively stable due to the strong electron-donating nature of this ligand, which limits their applicability. Here we use density functional theory calculations to show how simple addition of nitro and carboxylic acid groups to these cyclometalated complexes can engender a less oxidizable Fe(II) center while maintaining, or even improving, the favorable ligand field strength.


 Please wait while we load your content...
Please wait while we load your content...