Anti-cancer organoruthenium(ii) complexes and their interactions with cysteine and its analogues. A mass-spectrometric study†
Abstract
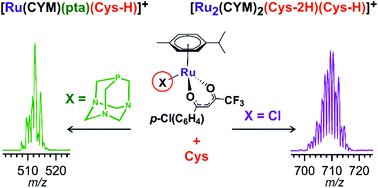
The ruthenium complexes [Ru(CYM)(p-Cl-dkt)(Cl)] (1), [Ru(CYM)(pta)(p-Cl-dkt)]PF6 (2), and [Ru(CYM)(pta)Cl2] (3, RAPTA-C) (CYM = para-cymene, p-Cl-dkt = 1-(4-chlorophenyl)-4,4,4-trifluorobutane-1,3-dione, pta = 1,3,5-triaza-7-phosphaadamantane) are biologically active and show anti-cancer activities, albeit with different mechanisms. To further understand these mechanisms, we compared their speciation in aqueous solutions with an amino acid (cysteine), with an amino acid derivative (N-acetylcysteine) and with a tripeptide (glutathione) by Mass Spectrometry (MS). Here, we show that all ruthenium complexes have high selectivity for cysteine and cysteine-derived molecules. On one hand, [Ru(CYM)(p-Cl-dkt)(Cl)] undergoes solvolysis in water and forms [Ru2(CYM)2(OH)3]+. Subsequently, all hydroxyl anions are exchanged by deprotonated cysteine. Infrared Photodissociation Spectroscopy (IRPD) showed that cysteine binds to the ruthenium atoms via the deprotonated thiol group and that sulfur bridges the ruthenium centers. On the other hand, the pta-bearing complexes remain monometallic and undergo only slow Cl or p-Cl-dkt exchange by deprotonated cysteine. Therefore, the pta ligand protects the ruthenium complexes from ligand exchange with water and from the formation of biruthenium clusters, possibly explaining why the mechanism of pta-bearing ruthenium complexes is not based on ROS production but on their reactivity as monometallic complexes.


 Please wait while we load your content...
Please wait while we load your content...