Tetrazastannoles versus distannadiazanes – a question of the tin(ii) source†
Abstract
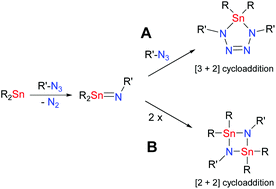
Various tin(II) compounds such as Mes*2Sn (Mes* = 2,4,6-tri-tert-butylphenyl), Sn[N(SiMe3)2]2 and TerSnCl (Ter = 2,6-bis(2,4,6-trimethylphenyl)phenyl) could be readily oxidised by organic azides to release N2, forming nitrogen-tin compounds. Depending on the used Sn(II) compound, the reactions with two equivalents of azide led to the formation of tetrazastannoles (R2N4SnR′2) or cyclo-distannadiazanes [R2Sn(μ-NR′)]2. The reactivity was independent of the electronic situation of the organic azide. Additionally, Mes*2Sn formed an amido-azido compound of the type R(R′)Sn(N(SiMe3)2)N3 in the presence of Me3SiN3. Presumably, the corresponding tetrazastannole was formed in the first step followed by a ring opening reaction. The same holds true for the reaction of Sn[N(SiMe3)2]2 with Me3SiN3 while TerSnCl showed no reaction in the presence of Me3SiN3, even after prolonged heating.
- This article is part of the themed collection: Inorganic chemistry of the p-block elements


 Please wait while we load your content...
Please wait while we load your content...