Synthesis and crystal structure of double-three ring (D3R)-type cage siloxanes modified with dimethylsilanol groups†
Abstract
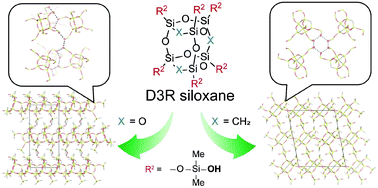
The controlled assembly of molecular building blocks enables the rational design of nanomaterials. In this study, two types of cage-type oligosiloxanes with double-three ring (D3R) structures are modified with dimethylsilanol groups to form supramolecular assemblies. One is the siloxane cage derived from Si(OEt)4 (denoted as the Q6 cage), and the other is the organosiloxane cage derived from (EtO)3Si–CH2–Si(OEt)3 (denoted as the T6 cage). The syntheses of the silanol-modified cages are performed in two steps: (i) dimethylsilylation of the corner Si–O− groups on the Q6 and T6 cages to introduce Si–H groups and (ii) subsequent oxidation of the Si–H groups to Si–OH groups. Dimethylsilylation of the cages is conducted at much lower temperatures (−94 and −78 °C for Q6 and T6 cages, respectively) than those used for conventional silylation, which is the key to suppressing the deterioration of the unstable D3R structure. The subsequent oxidation of the Si–H groups proceeds successfully, and the crystallization of these molecules is induced by the hydrogen bonds of the silanol groups. The crystal structure of the Q6 cage modified with dimethylsilanol groups can be regarded as a layered structure with tetrahydrofuran between the layers. In contrast, the T6 cage modified with dimethylsilanol groups assembled to form a more densely packed structure with no included solvent molecules. The differences between the crystal structures are discussed in terms of the shape of the cages. The insight into the effect of the shape of the cage on its assembly behavior will lead to the designable synthesis of crystalline siloxane-based materials.


 Please wait while we load your content...
Please wait while we load your content...