Reversible complexation of ammonia by breaking a manganese–manganese bond in a manganese carbonyl ethylenedithiolate complex: a theoretical study of an unusual type of Lewis acid†
Abstract
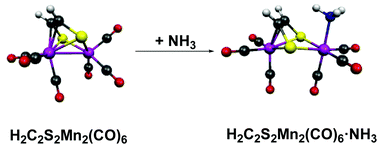
The reaction of Mn(CO)5Br with sodium ethylenedithiolate was reported in 1968 to give a dark red binuclear H2C2S2Mn2(CO)6 complex possessing the unusual property of complexing reversibly with ammonia to give a yellow H2C2S2Mn2(CO)6·NH3 adduct. In order to provide some insight into the nature of this adduct, density functional studies were performed on the H2C2S2Mn2(CO)n (n = 4 to 8) systems as well as their relevant ammonia and trimethylphosphine adducts. These theoretical studies support the structure of H2C2S2Mn2(CO)6 originally suggested 50 years ago involving the binding of the ethylenedithiolate C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond as well as the sulfur atoms to the Mn2 unit with a bonding Mn–Mn distance of ∼2.8 Å. Complexation of H2C2S2Mn2(CO)6 with NH3 or Me3P preserves the complexed C
C double bond as well as the sulfur atoms to the Mn2 unit with a bonding Mn–Mn distance of ∼2.8 Å. Complexation of H2C2S2Mn2(CO)6 with NH3 or Me3P preserves the complexed C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond of the ethylenedithiolate ligand but lengthens the Mn⋯Mn distance to a non-bonding ∼3.6 Å. Thus H2C2S2Mn2(CO)6 represents a novel type of Lewis acid where reversible complexation with Lewis bases involves the rupture of a metal–metal bond. Carbonyl dissociation energies in the H2C2S2Mn2(CO)n series account for the formation of the hexacarbonyl H2C2S2Mn2(CO)6 as the stable product from the Mn(CO)5Br/ethylenedithiolate reaction.
C double bond of the ethylenedithiolate ligand but lengthens the Mn⋯Mn distance to a non-bonding ∼3.6 Å. Thus H2C2S2Mn2(CO)6 represents a novel type of Lewis acid where reversible complexation with Lewis bases involves the rupture of a metal–metal bond. Carbonyl dissociation energies in the H2C2S2Mn2(CO)n series account for the formation of the hexacarbonyl H2C2S2Mn2(CO)6 as the stable product from the Mn(CO)5Br/ethylenedithiolate reaction.


 Please wait while we load your content...
Please wait while we load your content...