Benzoic acid as a selector–modulator in the synthesis of MIL-88B(Cr) and nano-MIL-101(Cr)†
Abstract
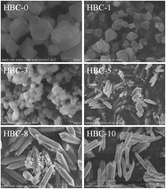
The concentration of benzoic acid was found to exercise efficient control over the formation of either MIL-101(Cr) or MIL-88B(Cr) under otherwise similar hydrothermal synthetic conditions. Nanocrystals of MIL-101(Cr) with ∼100 nm average size and excellent SBET = 3467 m2 g−1 are obtained at lower concentrations of benzoic acid, while at higher concentrations the microparticulated MIL-88B(Cr) product is formed. Hereby a new efficient synthetic method towards the elusive MIL-88B(Cr), yet reported only once without synthetic details, is proposed. The obtained MIL-88B(Cr) has an interesting and potentially valuable property of retaining its high-volume form (Vcell ∼ 2000 Å3) after thermal activation. The degassing of MIL-88B(Cr) in a vacuum at 250 °C yields a porous material with a SBET area of 1136 m2 g−1, which is around the theoretical maximum. The transition to the denser ‘closed’ form (Vcell ∼ 1500 Å3) occurs only at 350 °C, when all of the benzoate/benzoic acid, hindering the process, is removed.


 Please wait while we load your content...
Please wait while we load your content...