Fabrication of a full-spectrum-response Cu2(OH)2CO3/g-C3N4 heterojunction catalyst with outstanding photocatalytic H2O2 production performance via a self-sacrificial method
Abstract
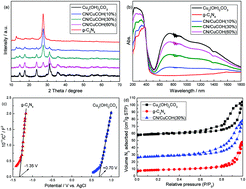
Over the past few decades, near infrared light (NIR), as an important part of sunlight, has seldom been utilized in photocatalytic reactions. In this work, a full-spectrum-response Cu2(OH)2CO3/g-C3N4 photocatalyst with outstanding photocatalytic H2O2 production performance was synthesized. XRD, UV-Vis, N2 adsorption, XPS, PL, EIS and EPR are used to characterize the as-prepared catalysts. As a light absorber from UV to NIR, Cu2(OH)2CO3 can form more photogenerated electrons to recombine the holes in g-C3N4 through the “Z-scheme” mechanism. The as-prepared Cu2(OH)2CO3/g-C3N4 photocatalyst shows the H2O2 equilibrium concentration of 8.9 mmol L−1, over 16 and 26.9 times higher than that of neat g-C3N4 and Cu2(OH)2CO3. According to the Z-scheme mechanism, a “two channel route” to form H2O2 is proposed for the Cu2(OH)2CO3/g-C3N4 heterojunction catalyst.


 Please wait while we load your content...
Please wait while we load your content...