Non-conventional synthesis and photophysical studies of platinum(ii) complexes with methylene bridged 2,2′-dipyridylamine derivatives†
Abstract
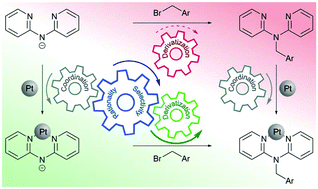
Methylene bridged 2,2′-dipyridylamine (dpa) derivatives and their metal complexes possess outstanding properties due to their inherent structural flexibility. Synthesis of such complexes typically involves derivatization of dpa followed by coordination on metals, and may not always be very efficient. In this work, an alternative synthetic approach, involving the derivatization step after – rather than prior to – coordination of dpa on metal center, is proposed and applied to synthesis of a number of platinum(II) complexes with substituted benzyldi(2-pyridyl)amines. Comparison with the more conventional synthetic route reveals greater efficiency and versatility of the proposed approach. The obtained complexes are not luminescent in solution at room temperature, but display blue phosphorescence emission (ca. 415 nm) with the lifetimes of μs order in glassy matrix at 77 K, with additional green (ca. 485 nm) and relatively long living (τ = 3.7 ms) emission in the case of iodine substituted derivative.


 Please wait while we load your content...
Please wait while we load your content...