Above room temperature spin crossover in thioamide-functionalised 2,6-bis(pyrazol-1-yl)pyridine iron(ii) complexes†
Abstract
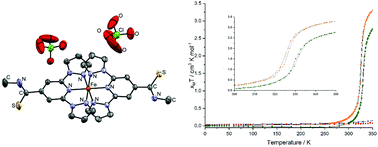
This work describes the synthesis of two novel functionalised 2,6-bis(pyrazol-1-yl)pyridine (bpp) ligands, namely 2,6-bis(pyrazol-1-yl)pyridine-4-carbothioamide (bppCSNH2) and N-methyl-2,6-bis(pyrazol-1-yl)pyridine-4-carbothioamide (bppCSNHMe). The corresponding solvated or non-solvated Fe(II) salts, [Fe(bppCSNH2)2]X2 and [Fe(bppCSNHMe)2]X2 (X = BF4− or ClO4−) were synthesised and their properties measured by SQUID magnetometry, Evans NMR, differential scanning calorimetry and single crystal X-ray diffraction. In the solid state [Fe(bppCSNH2)2]2+ salts persist in the low spin state below 350 K. The structure of [Fe(bppCSNH2)2](BF4)2·2MeNO2 shows a network of intermolecular interactions responsible for the low spin state stabilisation, relative to the prototypical [Fe(bpp)2]2+ spin crossover (SCO) salts. By contrast the complexes of bppCSNHMe both display abrupt SCO above 300 K. [Fe(bppCSNHMe)2](BF4)2·MeNO2 requires solvent loss before SCO can be observed centred at 332 K. The non-solvated [Fe(bppCSNHMe)2](ClO4)2 shows SCO centred at 325 K. Analysis of solvated and non-solvated crystal structures suggests that cooperativity is facilitated by thioamide-group interactions with neighbouring pyrazolyl and pyridyl moieties.


 Please wait while we load your content...
Please wait while we load your content...