The chemically reduced CuO–Co3O4 composite as a highly efficient electrocatalyst for oxygen evolution reaction in alkaline media†
Abstract
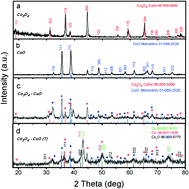
The fabrication of efficient, alkaline-stable and nonprecious electrocatalysts for the oxygen evolution reaction is highly needed; however, it is a challenging task. Herein, we report a noble metal-free advanced catalyst, i.e. the chemically reduced mixed transition metal oxide CuO–Co3O4 composite, with outstanding oxygen evolution reaction activity in alkaline media. Sodium borohydride (NaBH4) was used as a reducing agent for the mixed transition metal oxide CuO–Co3O4. The chemically reduced composite carried mixed valence states of Cu and Co, which played a dynamic role in driving an excellent oxygen evolution reaction process. The X-ray photo-electron spectroscopy (XPS) study confirmed high density of active sites in the treated sample with a large number of oxygen vacancies. The developed electrocatalyst showed the lowest overpotential of 144.5 mV vs. the reversible hydrogen electrode (RHE) to achieve the current density of 40 mA cm−2 and remained stable for 40 hours throughout the chronoamperometry test at the constant potential of 1.39 V vs. RHE. Moreover, the chemically reduced composite was highly durable. Electrochemical impedance spectroscopy (EIS) confirmed the low charge transfer resistance of 13.53 ohms for the chemically reduced composite, which was 50 and 26 times smaller than that of Co3O4 and untreated CuO–Co3O4, respectively. The electrochemically active surface area for the chemically reduced composite was found to be greater than that for pristine CuO, Co3O4 and untreated pristine CuO–Co3O4. These findings reveal the possibility of a new gateway for the capitalization of a chemically reduced sample into diverse energy storage and conversion systems such as lithium-ion batteries and supercapacitors.


 Please wait while we load your content...
Please wait while we load your content...