Octanoic acid hydrodeoxygenation over bifunctional Ni/Al-SBA-15 catalysts†
Abstract
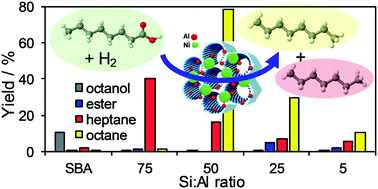
Hydroprocessing of fatty carboxylic acids from waste oil feedstocks, via hydrodeoxygenation (HDO), hydrodecarbonylation (HDC) or decarboxylation (DCX), is a promising route to produce hydrocarbon fuels. Bifunctional metal doped solid acids show potential for low temperature hydroprocessing, however few studies systematically explore the role of support acidity on catalyst performance. Here we report the liquid phase hydroprocessing of octanoic acid, a model aliphatic carboxylic acid, over bifunctional 5 wt% Ni/Al-SBA-15 mesoporous solid acids with Si : Al molar ratios spanning 5 to 75. The different hydroprocessing pathways show a strong dependence on support acidity at 13–20% iso-conversion, with selectivity to C8 alkane production by HDO increasing with support Brønsted acidity, whereas competing HDC or DCX to C7 alkanes decreases. 5 wt% Ni/Al-SBA-15 with a Si : Al ratio of 5 exhibits the highest Brønsted : Lewis ratio and selectivity to HDO products, but shows a low activity attributed to coking. In contrast, catalysts with intermediate acidity (Si : Al = 54) are more stable, exhibiting 95% conversion and 74% selectivity to C8 alkanes after 5 h reaction.


 Please wait while we load your content...
Please wait while we load your content...