Towards practical earth abundant reduction catalysis: design of improved catalysts for manganese catalysed hydrogenation†‡
Abstract
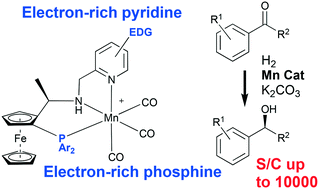
Manganese catalysts derived from tridentate P,N,N ligands can be activated easily using weak bases for both ketone and ester hydrogenations. Kinetic studies indicate the ketone hydrogenations are 0th order in acetophenone, positive order in hydrogen and 1st order in the catalyst. This implies that the rate determining step of the reaction was the activation of hydrogen. New ligand systems with varying donor strength were studied and it was possible to make the hydrogen activation significantly more efficient; a catalyst displaying around a 3-fold increase in initial turn-over frequencies for the hydrogenation of acetophenone relative to the parent system was discovered as a result of these kinetic investigations. Ester hydrogenations and ketone transfer hydrogenation (isopropanol as reductant) are first order for both the substrate and catalysts. Kinetic studies also gained insight into catalyst stability and identified a working range in which the catalyst is stable throughout the catalytic reaction (and a larger working range where high yields can still be achieved). The new more active catalyst, combining an electron-rich phosphine with an electron-rich pyridine is capable of hydrogenating acetophenone using as little as 0.01 mol% catalyst at 65 °C. In all, protocols for reduction of 21 ketones and 15 esters are described.


 Please wait while we load your content...
Please wait while we load your content...