Manganese PNP-pincer catalyzed isomerization of allylic/homo-allylic alcohols to ketones – activity, selectivity, efficiency†
Abstract
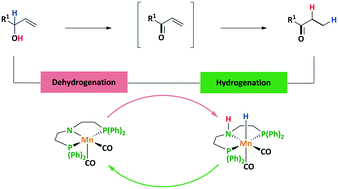
We report the first manganese catalyzed isomerization of allylic alcohols to produce the corresponding carbonyl compounds. The ligand plays a decisive role in the efficiency of this reaction. Very high conversions could be obtained using a solvent-free reaction system. A detailed DFT study reveals a self-dehydrogenation/hydrogenation reaction mechanism which was verified by the isolation of the α,β-unsaturated ketone as intermediate and a deuterium labeling experiment. It also provided a rationale for the observed selectivity and the higher efficiency of phenyl over isopropyl substitution.


 Please wait while we load your content...
Please wait while we load your content...