Polyoxometalates as alternative Mo precursors for methane dehydroaromatization on Mo/ZSM-5 and Mo/MCM-22 catalysts†
Abstract
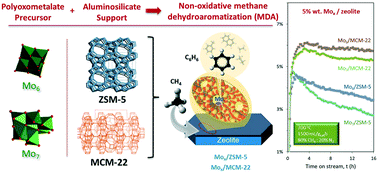
The conversion of methane into higher molecular weight hydrocarbons of greater added value has emerged as one of the grand challenges of the 21st century. The non-oxidative methane dehydroaromatization (hereafter MDA) reaction is a promising methane valorisation reaction since it transforms methane into added-value aromatics and olefins, namely benzene, naphthalene and ethylene. Molybdenum-promoted ZSM-5 zeolite has proven to be one of the most effective catalysts for MDA providing a shape-selective environment for the conversion of methane into benzene. However, one of the principle disadvantages of using aluminosilicates in the presence of methane is that the catalyst suffers from rapid deactivation induced by coke formation, which ultimately leads to a decrease in activity and aromatics selectivity, making the process unsuitable for large-scale industrial applications. Better control of the metal dispersion on the surface of the aluminosilicate supports represents a crucial factor to partially suppress catalyst coking and improve stability. Here we show how different molecular polyoxomolybdate (POM) anions can be used as alternative Mo precursors to conventional Mo salts for the preparation of catalysts for the MDA reaction. Molecular dynamics simulations and experimental testing were conducted to characterize the interphase interactions between polyoxomolybdates and zeolite surfaces at the atomistic level and to evaluate the MDA performance of different POM-based catalysts supported on ZSM-5 and MCM-22, respectively. The catalysts prepared using hexamolybdate anions, [Mo6O19]2−, were found to be more active and selective towards benzene than those employing the commercial heptamolybdate, [Mo7O24]6−. The Mo loading and dispersion of MoOx species were found to be the key factors leading to enhanced catalytic stability on ZSM-5 and MCM-22-based supports for MDA where the 5% Mo6/MCM-22 catalyst provided a constant aromatics yield above 7% for more than 18 hours time-on stream operating at 700 °C with a diluted methane flow under atmospheric pressure. The zeolitic catalysts prepared with the Mo6 precursor were found to be amongst the most promising MDA catalysts in the literature and the results of this study pave the way for the selection and use of different POMs as innovative metal precursors to formulate new catalysts and further improve the MDA reaction process.


 Please wait while we load your content...
Please wait while we load your content...