How the acido-basic properties of Mg silicates and clays govern the catalytic mechanism of transesterification reactions†
Abstract
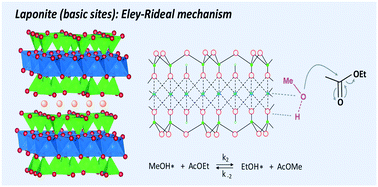
This study aims to investigate the relationship between the acido-basic properties of magnesium silicates and the mechanism of transesterification (TE) reactions in the liquid phase. Transesterification of ethyl acetate (AcOEt) with methanol (MeOH) was studied and the acido-basic properties of the catalysts were probed thanks to the model reaction of 2-methyl-but-3-yn-2-ol conversion. A commercial magnesium silicate (Magnesol®) and a clay (LAPONITE®) exhibit the best catalytic performances while they have different acido-basic properties: acid and basic sites are revealed for Magnesol® whereas LAPONITE® exhibits mainly basic sites. To highlight this difference in behaviours, the role of water in the catalytic activity of LAPONITE® was considered and compared to the results previously obtained in the case of Magnesol®. This study underlines that like on basic catalysts, LAPONITE® is poisoned by water molecules adsorbed on its surface. In contrast, in the case of Magnesol®, the presence of water adsorbed on the surface of this material is beneficial in terms of its catalytic activity. Furthermore, a kinetic study was performed in order to reveal the reaction mechanism involved in the TE on these two catalysts. According to the experimental kinetic data, the TE reaction on bifunctional catalysts such as Magnesol® obeys the Langmuir–Hinshelwood mechanism involving the adsorption of AcOEt on acid sites while MeOH on basic sites. Contrariwise, on mainly basic materials such as LAPONITE® the TE reaction obeys the Eley–Rideal mechanism, being the reaction of adsorbed MeOH with ethyl acetate in the liquid phase. Better performance of LAPONITE® in the TE of a longer carbon chain ester (ethyl butanoate) seems to confirm this kinetic study which shows that the ester does not need to adsorb.


 Please wait while we load your content...
Please wait while we load your content...