One step phenol synthesis from benzene catalysed by nickel(ii) complexes†
Abstract
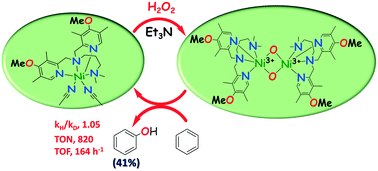
Nickel(II)complexes of N4-ligands have been synthesized and characterized as efficient catalysts for the hydroxylation of benzene using H2O2. All the complexes exhibited Ni2+ → Ni3+ oxidation potentials of around 0.966–1.051 V vs. Ag/Ag+ in acetonitrile. One of the complexes has been structurally characterized and adopted an octahedral coordination geometry around the nickel(II) center. The complexes catalysed direct benzene hydroxylation using H2O2 as an oxygen source and afforded phenol up to 41% with a turnover number (TON) of 820. This is unprecedentedly the highest catalytic efficiency achieved to date for benzene hydroxylation using 0.05 mol% catalyst loading and five equivalents of H2O2. The benzene hydroxylation reaction possibly proceeds via the key intermediate bis(μ-oxo)dinickel(III) species, which was characterized by HR-MS, vibrational and electronic spectral methods, for almost all complexes. The formation constant of the key intermediate was calculated to be 5.61–9.41 × 10−2 s−1 by following the appearance of an oxo-to-Ni(III) LMCT band at around 406–413 nm. The intermediates are found to be very short-lived (t1/2, 73–123 s). The geometry of one of the catalytically active intermediates was optimized by DFT and its spectral properties were calculated by TD-DFT calculations, which are comparable to experimental spectral data. The kinetic isotope effect (KIE) values (0.98–1.05) support the involvement of nickel-bound oxygen species as an intermediate. The isotope-labeling experiments using H218O2 showed 92.46% incorporation of 18O, revealing that H2O2 is the key oxygen supplier to form phenol. The catalytic efficiencies of complexes are strongly influenced by the geometrical configuration of intermediates, and stereoelectronic and steric properties, which are fine-tuned by the ligand architecture.
- This article is part of the themed collection: 2019 Catalysis Science & Technology HOT Articles


 Please wait while we load your content...
Please wait while we load your content...