Structural evolution of Ag/BN hybrids via a polyol-assisted fabrication process and their catalytic activity in CO oxidation†
Abstract
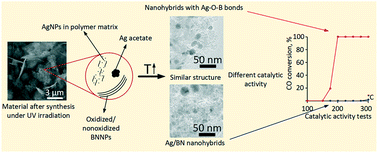
Herein we analyzed the chemical reactions occurring in the process of Ag/BN hybrid fabrication in polyethylene glycol (PEG) using h-BN nano- and microparticles (BNNPs and BNMPs) during AgNO3 decomposition under UV irradiation. Decomposition of AgNO3 causes formation of Ag nanoparticles (AgNPs) and PEG oxidation reaction, the products of which interact with Ag to form Ag acetate. The polymer matrix surrounding AgNPs prevents their growth during the fabrication process. Additional low temperature annealing leads to the decomposition of the Ag acetate phase, thereby increasing the volume fraction of AgNPs. In the case of the BNNPs, the AgNP size is smaller and their distribution is more homogeneous. BNNP surface oxidation was shown to be an important factor leading to enhanced catalytic activity of Ag/BN hybrids in the CO oxidation reaction: Tinitiation = 150 °C and T100 = 200 °C. Theoretical modelling suggests that the high catalytic activity of Ag/BN nanohybrids (NHs) can be explained by the formation of thin B–O–Ag layers.


 Please wait while we load your content...
Please wait while we load your content...