Mechanism of photocatalytic toluene oxidation with ZnWO4: a combined experimental and theoretical investigation
Abstract
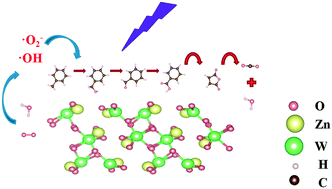
ZnWO4 catalysts are brilliant in the field of photocatalysis due to their excellent chemical stability and optical properties. In this work, we prepared pure ZnWO4 by a hydrothermal method, evaluated its ability to degrade toluene and elucidated the photocatalytic reaction mechanism. The results showed that ZnWO4 had excellent catalytic activity and stability. Combining the ESR spectroscopy spectra and DFT calculation results, ZnWO4 was found to adsorb a large amount of O2 and H2O, and activate them to produce active radicals participating in the oxidation reaction of toluene. The reactions to form benzyl alcohol and benzoic acid could be carried out spontaneously based on the Gibbs free energy calculation results and in situ DRIFTS spectra, which indicated that benzaldehyde can be easily converted to benzoic acid. The ring opening reaction is more likely to be carried out on benzoic acid. The findings of this work can extend the application of ZnWO4 materials and the understanding of the mechanism of photocatalytic degradation of toluene.


 Please wait while we load your content...
Please wait while we load your content...