Hydrolytic zinc metallopeptides using a computational multi-state design approach†
Abstract
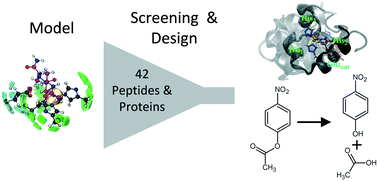
Hydrolytic zinc enzymes are common targets for protein design. The versatility of the zinc chemistry can be combined with the usage of small protein scaffolds for biocatalytic applications. Despite this, the computational design of metal-containing proteins remains challenging due to the need to properly model protein–metal interactions. We addressed these issues by developing a computational multi-state design approach of artificial zinc hydrolases based on small protein scaffolds. The zinc-finger peptide Sp1f2 was redesigned to accommodate a catalytic zinc centre and the villin headpiece C-terminal subdomain HP35 was de novo designed for metal-binding and catalytic activity. Both metallopeptides exhibited metal-induced folding (KZnP,app ≈ 2 × 105 M−1) and hydrolytic activity (k2 ≈ 0.1 M−1 s−1) towards an ester substrate. By focusing on the inherent flexibility of small proteins and their interactions with the metal ion by molecular dynamics simulations and spectroscopic studies, we identified current limitations on computational design of metalloenzymes and propose how these can be overcome by integrating information of protein–metal interactions in long time scale simulations.


 Please wait while we load your content...
Please wait while we load your content...