Entropic corrections for the evaluation of the catalytic activity in the Al(iii) catalysed formation of cyclic carbonates from CO2 and epoxides†‡
Abstract
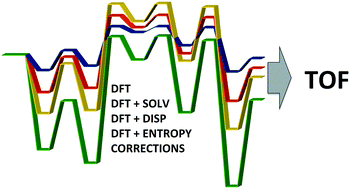
The reaction mechanism for formation of cyclic carbonates from CO2 and 1,2-epoxyhexane catalyzed by an [Al(amino-triphenolate)]/NBu4I binary system has been investigated by using density functional theory (DFT) based methods. A monometallic mechanism is proposed and the main steps of the reaction are described in detail. The energetic span model (δE) was used to theoretically determine the turnover frequencies (TOFs) of the catalytic cycle and to evaluate the efficiency of the aluminum complex in mediating the CO2 addition reaction. Our findings indicate that entropy changes in solution must be included in order to compute the TOF values in line with the experimental results.


 Please wait while we load your content...
Please wait while we load your content...