Ambient electrocatalytic nitrogen reduction on a MoO2/graphene hybrid: experimental and DFT studies†
Abstract
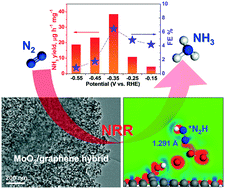
Rational design of effective electrocatalysts toward the N2 reduction reaction (NRR) is essential for achieving high-efficiency electrocatalytic NH3 synthesis. Herein, a hybrid catalyst of MoO2 nanoparticles on reduced graphene oxide (MoO2/RGO) was developed to catalyze the NRR under ambient conditions. Electrochemical measurements demonstrated that MoO2/RGO presented a high NRR activity with an NH3 yield of 37.4 μg h−1 mg−1 and a faradaic efficiency of 6.6% at −0.35 V (vs. RHE) in 0.1 M Na2SO4, comparable to or exceeding those of most reported NRR electrocatalysts. Density functional theory calculations disclosed that the MoO2/RGO hybrid possessed stronger electronic interactions with *N2H and donated more electrons from active Mo sites to *N2H relative to MoO2 alone, largely reducing the energy barrier for *N2H formation, which was the potential-determining step of the NRR process. These results highlight the promising potential of the hybridization strategy and MoO2/RGO hybrid for applications in ambient electrocatalytic NH3 synthesis.
- This article is part of the themed collection: 2019 Catalysis Science & Technology HOT Articles


 Please wait while we load your content...
Please wait while we load your content...