Network topology and cavity confinement-controlled diastereoselectivity in cyclopropanation reactions catalyzed by porphyrin-based MOFs†
Abstract
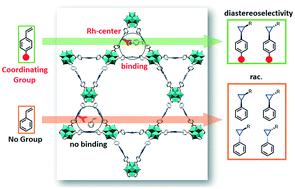
In this work, we show that the stereoselectivity of a reaction can be controlled by directing groups of substrates, by network topology and by local cavity confinement of metal–organic framework (MOF) catalysts. We applied the porphyrin-based PCN-224(Rh), which contains no stereocenters in the cyclopropanation reaction using ethyl diazoacetate (EDA) as carbene source. When styrene and other non-coordinating olefins are used as substrates, high activity, but no diastereoselectivity is observed. Interestingly, conversion of 4-amino- and 4-hydroxystyrene substrates occurs with high diastereomeric ratios (dr) of up to 23 : 1 (trans : cis). We attribute this to local pore confinement effects as a result of substrate coordination to neighboring Rh-centers, which position the olefin with respect to the active site, causing a break of local symmetry of the coordinated substrate. The effect of local pore confinement was improved by using PCN-222(Rh) as catalyst, which is a structural analog of PCN-224(Rh) with characteristic Kagomé topology featuring shorter Rh–Rh distances. A remarkable dr of 42 : 1 (trans : cis) was observed for 4-aminostyrene. In this case, the length of the substrate corresponds to the average distance between two neighboring Rh centers within the pores of PCN-222(Rh), which drastically boosts the diastereoselectivity. This work showcases how diastereomeric control can be achieved by favorable substrate–catalyst interactions and thoughtful adjustment of confined reaction space using porphyrin-based MOFs, in which stereocenters are inherently absent.


 Please wait while we load your content...
Please wait while we load your content...