Direct production of aromatics from syngas over a hybrid FeMn Fischer–Tropsch catalyst and HZSM-5 zeolite: local environment effect and mechanism-directed tuning of the aromatic selectivity†
Abstract
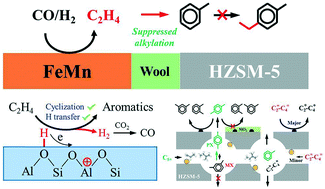
Direct conversion of syngas (CO/H2) into aromatics over tandem catalysts via the Fischer–Tropsch synthesis (FTS) route has attracted much attention because of its high one-pass conversion and milder reaction conditions. However, the complicated FTS product composition leads to more difficult mechanistic elucidation of aromatics formation compared to the methanol route. Herein, a series of strategies, such as covering external surface acid sites of HZSM-5 with a layer of inert SiO2, layered loading of an FeMn FTS catalyst and HZSM-5 zeolite in one reactor and two tandem reactors, and model reactions with ethylene and propylene over zeolite in a simulated FTS reaction environment, were applied to unveil the reaction mechanism and related factors affecting aromatic selectivity. It was found that different from the methanol route, the tandem FeMn–HZSM-5 catalyst produced aromatics mainly from the conversion of C5+ FT intermediates rather than light olefins (C2=–C4=). SiO2 coating could remarkably enhance the para-xylene (PX) selectivity due to hindering of its isomerization to major meta- and minor ortho-xylene (MX and OX) on the external surface acid sites of HZSM-5 by releasing the space confinement. The layered loading experiments in one reactor demonstrated that a farther distance from the FTS catalyst to zeolite largely increased the selectivity to BTX (benzene, toluene and xylene) in total aromatics from 48.3% to 68.9% on a carbon molar basis by suppressing the alkylation reaction of benzene and toluene with ethylene due to an obviously lower local ethylene concentration on HZSM-5. FeMn and HZSM-5 with layered loading in two tandem reactors confirmed that light olefins (C2=–C4=) were mainly hydrogenated and isomerized into paraffins. The model reactions with ethylene and propylene in a simulated local FTS reaction environment revealed that the presence of H2O and CO2in situ produced from the FTS reaction played a crucial role in promoting the aromatics formation at obviously lower reaction temperature which resulted from the enhancing acid strength and the driving H transfer to facilitate the cyclization and dehydrogenation reaction. This study provides a clear perspective for the aromatics formation mechanism and the tuning of the aromatic selectivity.


 Please wait while we load your content...
Please wait while we load your content...