Au2+ cannot catalyze conversion of methane to ethene at low temperature
Abstract
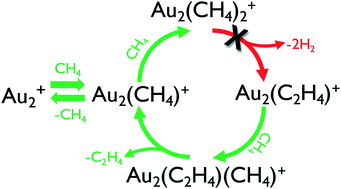
The previously reported conversion of methane to ethene catalyzed by Au2+ at thermal energies, at odds with established thermodynamics, is investigated through a combination of experiments under both single-collision conditions using a guided ion beam tandem mass spectrometer (GIBMS) apparatus and at higher pressures using a selected-ion flow tube (SIFT) apparatus, as well as through density functional calculations. Production of Au2(C2H4)+ (or m/z 422) or Au2(C2D4)+ (or m/z 426) is significantly lower in the higher pressure SIFT experiments relative to previously reported results. The amount observed is consistent with a pathway initiating from a small fraction of a reactive species, potentially electronically excited Au2+* or Au2O+, isobaric with Au2(CH4)+. Extensive theoretical exploration of the potential energy surface for Au2(CH4)+ + CH4 shows no low-energy pathway that is consistent with ethene formation, with prohibitive barriers of 1–2 eV calculated along all identified reaction coordinates, consistent with previous calculations. GIBMS data do show the production of m/z 422 in the reaction of Au2(CH4)+ + CH4, consistent with the proposed key intermediate, but also provide evidence that this observed species is Au2(CO)+ arising from Au2O+, not Au2(C2H4)+ arising from Au2(CH4)+. The present results are consistent with the established thermochemistry for methane-to-ethene conversion, which unambiguously demonstrates that such conversion cannot proceed at thermal energies regardless of the presence of Au2+.
- This article is part of the themed collection: 2019 Catalysis Science & Technology HOT Articles


 Please wait while we load your content...
Please wait while we load your content...