Iron carbide or iron carbide/cobalt nanoparticles for magnetically-induced CO2 hydrogenation over Ni/SiRAlOx catalysts†
Abstract
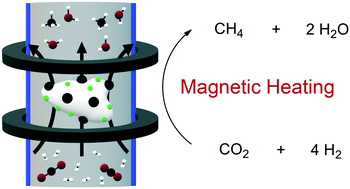
Magnetic nanoparticles have recently been used as heating agents in heterogeneously catalyzed reactions such as CO2 hydrogenation into methane. In the present work, we explore the potential of heating agents presenting different heating properties in the activation of a supported catalyst based on nickel nanoparticles (NPs) (Ni/SiRAlOx) for the CO2 methanation under continuous flow. Two types of Fe2.2C NPs presenting distinct heating properties have been tested. For Fe2.2C NPs displaying lower heating powers of ca. 1 kW g−1, an activation of the catalyst at a magnetic field amplitude of 80 mT is necessary to achieve high conversion and selectivity, this activation step being attributed to a partial sintering of the Ni NPs. When using Fe2.2C NPs displaying much higher heating powers of 2 kW g−1 as heating agents, the magnetic field amplitude required to activate the catalyst can be reduced to 48 mT. Finally, we demonstrate that hard magnetic materials displaying very low heating power but high Curie temperatures, such as Co nanorods (NRs), can be used as relay heating agents when mixed with small amounts of softer materials with high heating power. Thus, by mixing Co NRs with Fe2.2C NPs, excellent catalytic performances (90% of CO2 and 100% CH4 selectivity) have been obtained after applying only 32 mT to trigger the reaction. Once the reaction initiated, these performances are maintained even after lowering the magnetic field to 16 mT, which is advantageous in terms of energy consumption.


 Please wait while we load your content...
Please wait while we load your content...