Bimetallic NiCo/CNF encapsulated in a N-doped carbon shell as an electrocatalyst for Zn–air batteries and water splitting†
Abstract
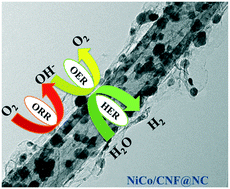
Exploring cheap and efficient electrocatalysts for the oxygen reduction reaction (ORR), oxygen evolution reaction (OER) and hydrogen evolution reaction (HER) as alternatives to precious metal-based electrocatalysts is crucial in energy storage and conversion devices. Herein, we report carbon nanofiber supported NiCo nanoalloys encapsulated in a N-doped carbon, NiCo/CNF@NC catalyst, with multifunctional activities. Benefiting from the unique hybrid structure and synergistic coupling between nanoalloys and N-doped carbon which can effectively inhibit the aggregation and detachment of nanoparticles while generating additional active sites, the resultant catalyst demonstrates remarkable ORR, OER and HER performances. NiCo/CNF@NC displays ORR performance with a comparable onset potential of 1.00 V and superior operational durability than the commercial Pt/C catalyst. Additionally, the NiCo/CNF@NC catalyst exhibits OER activity with an overpotential of 0.40 V at 10 mA cm−2 which is close to that of RuO2. The Zn–air battery based on the NiCo/CNF@NC catalyst delivers a specific capacity of 764 mA h g−1Zn with an energy density of 871 W h kg−1Zn at a current density of 20 mA cm−2. Moreover, the electrically rechargeable Zn–air battery operates with stable cyclic performance for more than 130 cycles. The water splitting device made of symmetric NiCo/CNF@NC catalyst coated nickel foam (NiCo/CNF@NC/NiF) electrodes has demonstrated an overall water splitting performance of 1.54 V at 10 mA cm−2 at a high catalyst loading of 4.6 mg cm−2.


 Please wait while we load your content...
Please wait while we load your content...