Metal-free gem selective dimerization of terminal alkynes catalyzed by a pyridonate borane complex†
Abstract
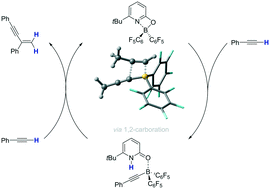
A metal free gem selective dimerization of terminal alkynes catalyzed by a pyridonate borane complex is described. Each individual step of the catalytic cycle was verified experimentally and a protocol for the catalytic reaction was developed. The mechanism of the reaction was further investigated by DFT and DLPNO-CCSD(T) computations. The catalytic transformation commences with C–H cleavage by a boroxypyridine that displays frustrated Lewis pair reactivity. The pyridone borane complex that forms upon C–H cleavage dissociates into a pyridone and an alkynylborane. An unprecedented 1,2-carboboration and a protodeborylation effected by the pyridone yield the 1,3-enyne and complete the catalytic cycle. The change in the coordination mode of the boroxypyridine upon C–H cleavage, described by the term boron-ligand cooperation, enables the dissociation of the formed pyridone borane complex and the 1,2-carboboration and is thus vital for the catalytic reaction.


 Please wait while we load your content...
Please wait while we load your content...