Graphitic carbon nitride/CoTPP type-II heterostructures with significantly enhanced photocatalytic hydrogen evolution†
Abstract
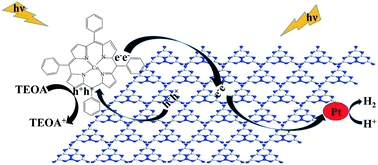
Graphitic carbon nitride (g-C3N4) is among the most promising metal-free photocatalysts for hydrogen (H2) evolution from water splitting under visible-light irradiation. However, the fast recombination rate of its photogenerated electron–hole pairs limits the photocatalytic activity of g-C3N4. Herein, we have developed highly efficient visible light driven C3N4/CoTPP type-II heterostructures by decorating cobalt(II) meso-tetraphenylporphine (CoTPP) on the surface of the g-C3N4 catalyst through π–π supramolecular interaction. The introduction of CoTPP enhances the migration and transfer of photogenerated charge carriers and inhibits the recombination of electron–hole pairs through the formation of C3N4/CoTPP type-II heterojunctions. The C3N4/CoTPP heterostructured photocatalyst exhibits efficient spatial separation of photogenerated electrons and holes due to the well-matched overlapping band structures of two semiconductors, thus facilitating more electrons to be released for H2 evolution. When 4 wt% (weight ratio) CoTPP is loaded onto g-C3N4, the H2 evolution rate of the C3N4/CoTPP heterostructured photocatalyst is significantly increased up to 46.93 μmol h−1 under visible light irradiation (λ ≥ 420 nm), which is 2.73 times higher than that of bare g-C3N4 (17.21 μmol h−1). Moreover, the C3N4/4 wt% CoTPP type-II heterostructured photocatalyst shows good stability for H2 evolution after five consecutive cycles of continuous irradiation of up to 20 h. Conclusively, the g-C3N4 photocatalyst coupled with CoTPP conjugated molecules substantially improves the photocatalytic performance and stability for H2 evolution from water under visible light irradiation. This novel strategy sheds light on developing other highly efficient photocatalytic systems for clean energy production.


 Please wait while we load your content...
Please wait while we load your content...