Insights into the CO2 deoxygenation to CO over oxygen vacancies of CeO2†
Abstract
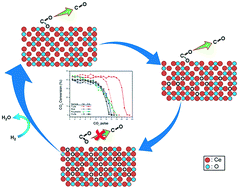
Cerium oxide (CeO2) is known to exhibit oxygen vacancy enhanced catalytic activity by itself or as a support. The particle shape effect on CO or VOC oxidation is also documented. This study reports the CO2 deoxygenation to CO over oxygen vacancies of CeO2 of different shapes, which can be a viable approach for CO2 utilization. A sequential experimental approach using a combination of TPR (temperature-programmed reduction under H2 up to 900 °C), TPO (temperature-programmed oxidation under 20% O2 up to 900 °C), and CO2 pulse reaction is performed. Tube and polyhedra CeO2 exhibit shape stability after TPR while cube and rod turn into polyhedral grains. Within a TPR-TPO-TPR or a TPR-CO2-TPR-CO2 test sequence, the tube sample shows the best reversibility in oxygen vacancy formation (based on the H2 consumption ratio in the two TPRs) and the highest oxygen vacancy capacity (based on the second TPR) among the four samples. The CO2 deoxygenation to CO occurs over the four TPR-treated samples and the initial per pulse CO2 conversion is steady, implying that the surface reaction governs the rate and that the diffusion of stripped oxygen into the bulk phase is relatively fast. The deoxygenation reactivity is reproducible in the two CO2 pulsing tests in TPR-CO2-TPR-CO2 experiments, indicating the importance of reversibly generated oxygen vacancies. The results also indicate that the deoxygenation activity of surface oxygen vacancies can be influenced by subsurface vacancies.


 Please wait while we load your content...
Please wait while we load your content...