Atom-economical regioselective Ni-catalyzed hydroborylative cyclization of enynes: development and mechanism†
Abstract
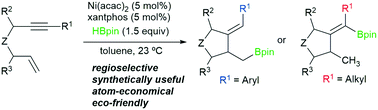
We report a full study on the novel regioselective Ni-catalyzed hydroborylative cyclization of enynes using HBpin as the borylation agent. Alkyl and alkenyl boronates can be obtained depending on the substituent on the alkyne. The reaction takes place under smooth conditions with an inexpensive catalytic Ni-based system, constituting a fully atom-economic eco-friendly method. This process shows a broad scope, allowing the formation of carbo- and heterocycles in moderate to good yields. The utility of the resulting boronates is also illustrated. We have studied the reaction mechanism both experimentally and computationally. The process involves initial oxidative cyclometalation to Ni(0)(xantphos) species followed by the key C–B bond formation through σ-bond metathesis and reductive elimination.


 Please wait while we load your content...
Please wait while we load your content...