Unveiling the mechanism and regioselectivity of iron-dipyrrinato-catalyzed intramolecular C(sp3)–H amination of alkyl azides†
Abstract
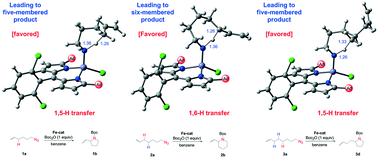
Iron-catalyzed direct amination of aliphatic C(sp3)–H bonds developed by Betley et al. (Science, 2013, 340, 591–595) is an efficient synthetic method to access a range of substituted pyrrolidines. Herein, we conducted density functional theory (DFT) calculations to explore the mechanism and origins of regioselectivity of this remarkable C(sp3)−H amination using an iron-dipyrrinato catalyst. Computational results show that iron-catalyzed C(sp3)–H amination occurs via the following phases: (a) ligand–substrate exchange offering the active Fe(II) catalyst; (b) oxidation of the Fe(II) catalyst to an Fe(III)-nitrene radical species using the alkyl azide substrate with the release of N2 molecules; (c) intramolecular H-abstraction (C⋯H⋯N) affording an alkyl radical and an Fe(III)-iminyl radical; and (d) radical rebound leading to a N-heterocyclic compound, which reacts with Boc2O to avoid product inhibition via a highly exergonic reaction affording an N-protected amine and regenerates the active Fe(II) catalyst by coordination with another alkyl azide substrate. Owing to the effortless nature of the radical rebound process, the calculations reveal that the H-abstraction step determines the regioselectivity of amination, with which arising mainly from a combination of radical stability and ring strain. The results demonstrate rich experimental–theoretical synergy and provide useful insights for further development of site-selective C–H functionalization reactions.


 Please wait while we load your content...
Please wait while we load your content...