Effect of Ce and La dopants in Co3O4 nanorods on the catalytic activity of CO and C3H6 oxidation†
Abstract
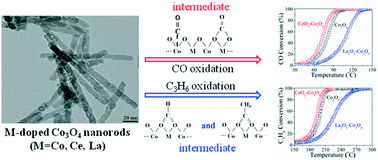
Ce and La doped Co3O4 nanorod catalysts were prepared and their catalytic activity for CO and C3H6 oxidation reactions was examined. XRD, TEM, XPS, TPR, TGA and in situ DRIFTS have been used to characterize structural properties, reducibility, mobility of adsorbed oxygen and lattice oxygen, and surface intermediates for both CO and C3H6 oxidation. When doping with either Ce or La, the cations occupy the Co2+ sites in Co3O4. XPS, TPR, and TGA characterization showed that the Ce dopant decreased the ratio of surface adsorbed oxygen to lattice oxygen, and facilitated the extraction of adsorbed oxygen and lattice oxygen with hydrogen from CeO2–Co3O4 in comparison to Co3O4, while the La dopant caused the opposite effect. The light-off performance for both CO and C3H6 oxidations has been improved due to Ce doping on Co3O4, while the doping of La actually inhibited the oxidation activity of the Co3O4 catalyst. Similar catalytic activity facilitation and inhibition of Ce and La dopants were also observed for the simultaneous oxidation of CO, C3H6, and NO under the conditions of simulated diesel exhaust. The results of in situ DRIFTS suggested that bidentate carbonate species could be the key intermediates of CO oxidation, which would further react with CO to form CO2. The results of DRIFTS showed that the mobility of lattice oxygen plays an important role in the C3H6 oxidation. A mechanism was proposed where the formate and acetate species could be the reaction intermediates of C3H6 oxidation, and these intermediates are more stable on La2O3–Co3O4 than on Co3O4 and CeO2–Co3O4 catalysts.


 Please wait while we load your content...
Please wait while we load your content...