Hydroboration of terminal olefins with pinacolborane catalyzed by new 2-iminopyrrolyl iron(ii) complexes†
Abstract
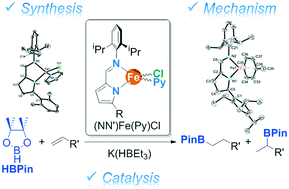
Four paramagnetic 14-electron tetracoordinated Fe(II) complexes of 5-substituted-2-iminopyrrolyl ligands of the type [Fe{κ2N,N′-5-R-NC4H2-2-C(H)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(2,6-iPr2-C6H3)}(Py)Cl], with R = 2,6-Me2-C6H3 (1a), 2,4,6-iPr3-C6H2 (1b), 2,4,6-Ph3-C6H3 (1c) and CPh3 (1d), were synthesized in moderate yields by reacting the respective 5-substituted-2-iminopyrrolyl potassium salts KLa-d with FeCl2(Py)4 in toluene. Complexes 1a–d were characterized by 1H NMR, FTIR spectroscopies, elemental analysis and by the Evans method, the corresponding effective magnetic moments showing a high-spin electronic nature. X-ray diffraction studies on complexes 1a and 1c showed distorted tetrahedral coordination geometries. Complexes 1a–c, activated with K(HBEt3), were efficient catalyst systems for the hydroboration of several terminal alkenes with pinacolborane in good to high yields (50–90%). This system mainly yielded the respective anti-Markovnikov addition products, except when styrenes were used. A screening of the hydroboration of styrene catalyzed by complexes 1a–c activated with K(HBEt3) showed that the selectivity in the Markovnikov product increased with increasing steric bulkiness of the R group, exhibiting selectivities up to 91%. Additionally, the stoichiometric reaction of complex 1b with K(HBEt3) over 30 minutes yielded the mixture of hydride species 2 and 22 (mixture I). On the other hand, when reacting the same components over 16 h, the Fe(I) complex 3 was also identified in the mixture, in addition to 2 + 22 (mixture II). These mixtures were characterized in solution by the Evans method and in the solid state by elemental analysis, 57Fe Mössbauer and FTIR spectroscopies, compounds 22 and 3 being also analyzed by X-ray diffraction. These results suggest that the corresponding catalytic cycle follows the borane oxidative addition route to a Fe(I) species.
N(2,6-iPr2-C6H3)}(Py)Cl], with R = 2,6-Me2-C6H3 (1a), 2,4,6-iPr3-C6H2 (1b), 2,4,6-Ph3-C6H3 (1c) and CPh3 (1d), were synthesized in moderate yields by reacting the respective 5-substituted-2-iminopyrrolyl potassium salts KLa-d with FeCl2(Py)4 in toluene. Complexes 1a–d were characterized by 1H NMR, FTIR spectroscopies, elemental analysis and by the Evans method, the corresponding effective magnetic moments showing a high-spin electronic nature. X-ray diffraction studies on complexes 1a and 1c showed distorted tetrahedral coordination geometries. Complexes 1a–c, activated with K(HBEt3), were efficient catalyst systems for the hydroboration of several terminal alkenes with pinacolborane in good to high yields (50–90%). This system mainly yielded the respective anti-Markovnikov addition products, except when styrenes were used. A screening of the hydroboration of styrene catalyzed by complexes 1a–c activated with K(HBEt3) showed that the selectivity in the Markovnikov product increased with increasing steric bulkiness of the R group, exhibiting selectivities up to 91%. Additionally, the stoichiometric reaction of complex 1b with K(HBEt3) over 30 minutes yielded the mixture of hydride species 2 and 22 (mixture I). On the other hand, when reacting the same components over 16 h, the Fe(I) complex 3 was also identified in the mixture, in addition to 2 + 22 (mixture II). These mixtures were characterized in solution by the Evans method and in the solid state by elemental analysis, 57Fe Mössbauer and FTIR spectroscopies, compounds 22 and 3 being also analyzed by X-ray diffraction. These results suggest that the corresponding catalytic cycle follows the borane oxidative addition route to a Fe(I) species.
- This article is part of the themed collection: 2019 Catalysis Science & Technology HOT Articles


 Please wait while we load your content...
Please wait while we load your content...