The role of weak Lewis acid sites for methanol thiolation†
Abstract
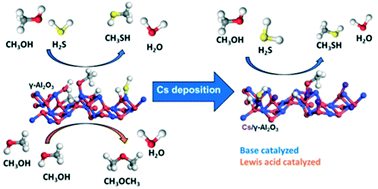
Weak Lewis acid sites combined with strong base sites of Cs+ supported on WS2 and γ-Al2O3 act as active sites in the thiolation of methanol. The acid–base pairs dissociate methanol upon adsorption. The formed surface alcoholate and the corresponding sulfuryl groups enable the substitution of oxygen for sulfur in a Langmuir–Hinshelwood mechanism. Stronger Lewis acid sites catalyze dimethyl ether formation via the Eley–Rideal mechanism in which methoxy groups react with gas phase methanol. The results demonstrate the importance of adjusting the acid–base strength in oxides to selectively catalyze substitution reactions.


 Please wait while we load your content...
Please wait while we load your content...