Rare-earth ion exchanged Cu-SSZ-13 zeolite from organotemplate-free synthesis with enhanced hydrothermal stability in NH3-SCR of NOx†
Abstract
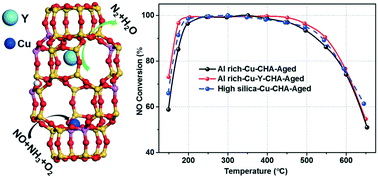
The relatively low hydrothermal stability of Al-rich Cu-SSZ-13 catalysts hinders their practical application in ammonia selective catalytic reduction (NH3-SCR) reaction. Rare-earth ions were introduced into the Al-rich SSZ-13 zeolite using an organotemplate-free synthesis prior to the exchange of Cu2+ ions. Among the rare-earth ions tested (Ce, La, Sm, Y, Yb), Y shows significant enhancement of the hydrothermal stability and NH3-SCR activities after severe hydrothermal aging at 800 °C for 16 h when compared with Cu-SSZ-13 without Y. Cu–Y-SSZ-13 catalysts with various amounts of Y were prepared, and it is found that with increasing Y content, the low temperature NO conversions can be improved even after hydrothermal aging. SEM-EDX analysis together with two-dimensional multiple quantum magic-angle-spinning nuclear magnetic resonance (23Na MQ MAS NMR) confirms that the Y ions are successfully incorporated into the ion-exchange sites of the SSZ-13 zeolite. Results from 27Al MAS, 29Si MAS NMR, temperature-programmed desorption of ammonia (NH3-TPD) and quantitative 1H MAS NMR indicate that Y can stabilize the framework Al and also preserve the Brønsted acid sites in the Al-rich SSZ-13 zeolite. The hydrogen temperature programmed reduction (H2-TPR), ultraviolet-visible-near infrared spectroscopy (UV-vis-NIR) and diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) of nitric oxide (NO) or NH3 adsorption demonstrate that introduction of Y ions causes Cu2+ ions to preferentially occupy the 6-MR, which has high hydrothermal stability. However, too much of Y may lead to activity loss at both low and high temperatures. The optimized Al-rich Cu–Y-SSZ-13 with 2.8 wt% of copper (Cu) and 1.3 wt% of Y displays almost the same deNOx activities as the conventional organotemplated high silica Cu-SSZ-13 catalyst in a wide reaction temperature window of 150–650 °C after severe hydrothermal treatment. Rare-earth ions could be an effective additive for Cu-SSZ-13 catalysts to further improve their hydrothermal stability for practical applications.


 Please wait while we load your content...
Please wait while we load your content...