A dimer path for CO dissociation on PtSn†
Abstract
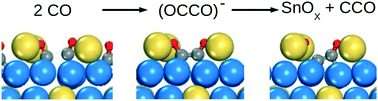
Density functional theory calculations are used to investigate CO adsorption, dissociation and SnOX formation on Pt3Sn. We find that direct CO dissociation is prevented by high activation energies. An energetically feasible path is instead CO dimer formation followed by C–O bond cleavage. Dimers are formed in the presence of Sn adatoms which effectively stabilize anionic OCCO− species. The presence of Sn adatoms is crucial as dimers are unstable on Pt-only systems. The proposed mechanism may explain recent experimental observations of SnOX and C–C formation as PtxSn is exposed to CO.


 Please wait while we load your content...
Please wait while we load your content...