Investigating homogeneous Co/Br−/H2O2 catalysed oxidation of lignin model compounds in acetic acid†
Abstract
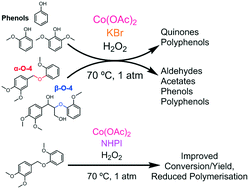
Oxidation of α-O-4, β-O-4 and monomeric lignin model compounds by Co/Br−/H2O2 in acetic acid at 70 °C was investigated. Co and Br− were introduced as cobalt acetate tetrahydrate and KBr respectively. The degree of methoxylation of the substrate was found to affect its reactivity. For the α-O-4 model compounds, increased methoxylation of the benzyl moiety influenced product selectivity, while increased methoxylation of the phenolic moiety increased substrate conversion. The β-O-4 model compounds exhibited similar conversions to the α-O-4 models, but afforded a lesser amount of monomeric products. The formation of phenol and guaiacol from α-O-4 bond cleavage inhibited substrate conversion and sequestered oxidation products due to the formation of phenoxy radicals and polyguaiacols. Similar to the α-O-4 model compounds, increased methoxylation of the monomers changed the types of products formed, from polyphenols (phenol and guaiacol) to quinones (syringol). The behaviour of syringol was explored extensively, revealing that the corresponding 1,4-hydroquinone strongly inhibited syringol oxidation, and the syringol oxidation product, 4,4′-diphenoquinone, was susceptible to over-oxidation. The deleterious effects of phenols on oxidation of an α-O-4 model could be reduced by substitution of the Br− co-catalyst with N-hydroxyphthalimide (NHPI), improving substrate conversion and product selectivity.


 Please wait while we load your content...
Please wait while we load your content...