Mechanical insights into the oxidative cleavage of resveratrol catalyzed by dioxygenase NOV1 from Novosphingobium aromaticivorans: confirmation of dioxygenase mechanism by QM/MM calculations†
Abstract
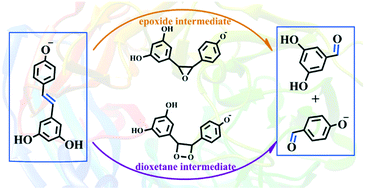
NOV1 is a stilbene cleavage oxygenase (SCO) responsible for the oxidative cleavage of the central double bond of stilbenes, forming two phenolic aldehydes. In the active site of NOV1, the iron cofactor is coordinated by four histidines and no negatively charged ligand, which is unusual for iron-containing enzymes. Here, on the basis of the recently obtained crystal structure of NOV1, we performed quantum mechanics/molecular mechanics (QM/MM) calculations to elucidate the reaction mechanism of NOV1. According to our calculations, two binding modes of O2 to Fe(II) (end-on and side-on modes) have been recognized, and the end-on mode is suitable for catalysis. The side-on mode, which has been obtained from the crystal, is an inactive form, which should first change to the end-on mode to initiate the catalytic reaction. For the reactant in end-on mode, the triplet and quintet states correspond to very similar energies. Of the three considered pathways, two pathways (path_a and path_b1, which differ only in the order of the first C–O bond formation) involving the dioxetane intermediate are calculated to be favorable, which can confirm the dioxygenase mechanism of NOV1. However, the pathway (path_b2) involving the epoxide intermediate corresponds to high energy barriers for the formation of the second C–O bond (27.49 and 31.56 kcal mol−1 on the triplet and quintet state surfaces) and can be ruled out. However, in path_b2, the formation of the epoxide intermediate is easy, which may react with the solvent water (non-enzymatic reaction) to form the by-product. In addition, the different protonation states of 4′-OH of the substrate and K134, as well as the coordination of additional water molecules with the iron, do not influence the reaction barrier.


 Please wait while we load your content...
Please wait while we load your content...