Effect of ammonia on cobalt Fischer–Tropsch synthesis catalysts: a surface science approach
Abstract
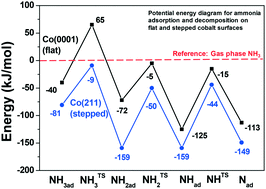
Ammonia adsorption and decomposition on defect-rich hcp-Co(0001) surfaces were investigated under ultra-high vacuum conditions in order to provide a fundamental explanation for industrially observed ammonia poisoning of cobalt based Fischer–Tropsch synthesis (FTS) catalysts. Temperature-programmed desorption, infrared spectroscopy and work function measurements indicate that undercoordinated sites bind ammonia stronger than sites on flat Co(0001), and they also induce its dehydrogenation. Density functional theory calculations were employed to explore the reactivity of defective Co surfaces using the fcc-Co(211) as a model. The results indicate that the decomposition products (NHx) adsorb strongly on or around the step site on fcc-Co(211). We find that NH (+2Had), adsorbed in the threefold site on the upper terrace, is equally stable as NH2 (+Had), adsorbed in the bridge position at the step edge, both being significantly more stable than the equivalent species adsorbed on the flat Co(0001). The calculated activation barriers for NH3,ad dehydrogenation steps are in reasonable agreement with the barriers obtained by fitting experimental data. Based on these fundamental insights, poisoning of cobalt nanoparticles during FTS by NH3 contaminants can be linked mainly to the blocking of undercoordinated sites by strongly adsorbed NH2 species.


 Please wait while we load your content...
Please wait while we load your content...