HCl and O2 co-activated bis(8-quinolinolato) oxovanadium(iv) complexes as efficient photoactive species for visible light-driven oxidation of cyclohexane to KA oil†
Abstract
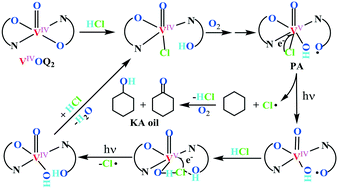
This paper reports three bis(8-quinolinolato) oxovanadium(IV) complexes that are active for visible light-induced cyclohexane oxygenation under O2 (1 atm) in acetonitrile with HCl participation, achieving greater than 18% conversion and 86% selectivity for KA oil (cyclohexanone and cyclohexanol), along with a small amount of chlorinated product. Notably, these VIVOQ2 complexes are not only basically comparable to VIVO(acac)2 and some typical VV-compounds in photocatalytic activity but are also more selective for KA oil. Spectral characterizations (XPS, FT-IR and UV-vis) and DFT calculations support that VIVOQ2 complexes can be co-activated by HCl and O2 to form the VVO(OH)-containing photoactive (PA) species with one pendant ligand's phenoxyl radical and an axially coordinated chlorine, which are responsible for the present photocatalytic oxidation. Noteworthily, increasing the Cl-substituents of VIVOQ2 complexes and especially the amount of water in the reaction system obviously decelerated the generation of such PA species, which significantly improved the selectivity for KA oil.


 Please wait while we load your content...
Please wait while we load your content...