New keys for old locks: carborane-containing drugs as platforms for mechanism-based therapies
Abstract
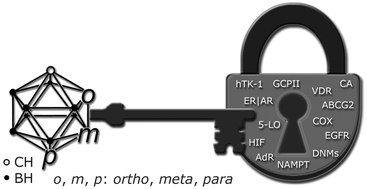
Icosahedral carboranes in medicine are still an emerging class of compounds with potential beneficial applications in drug design. These highly hydrophobic clusters are potential “new keys for old locks” which open up an exciting field of research for well-known, but challenging important therapeutic substrates, as demonstrated by the numerous examples discussed in this review.
- This article is part of the themed collection: Contemporary Research in Boron Chemistry


 Please wait while we load your content...
Please wait while we load your content...