Boronic acid catalysis
Abstract
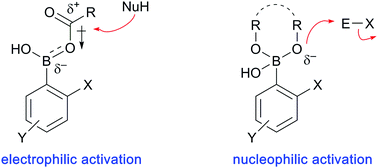
Although boronic acids are recognized primarily for their utility as reagents in transition metal-catalyzed transformations, other applications are emerging, including their use as reaction catalysts. Few methods are available for the catalytic activation of hydroxy functional groups as a way to promote their direct transformation into useful products under mild conditions. To this end, the ability of boronic acids to form reversible covalent bonds with hydroxy groups can be exploited to enable both electrophilic and nucleophilic modes of activation in various organic reactions. Using the concept of boronic acid catalysis (BAC), electrophilic activation of carboxylic acids leads to the formation of amides from amines, as well as cycloadditions and conjugate additions with unsaturated carboxylic acids. Alcohols can also be activated with boronic acid catalysts to form carbocation intermediates that can be trapped in selective Friedel–Crafts-type reactions with arenes and other nucleophiles. On the other hand, diols and saccharides can form tetrahedral adducts with boronic acids, which increases their nucleophilic character towards electrophiles. Altogether, BAC imparts mild and selective reaction conditions that display high atom-economy by circumventing the need for wasteful stoichiometric activation of hydroxy groups into halides or sulfonates.
- This article is part of the themed collection: Contemporary Research in Boron Chemistry


 Please wait while we load your content...
Please wait while we load your content...